As spring gardening approaches, a new contender has entered the fray—the genetically modified (GM) Purple Tomato. Unlike its GM predecessors, the GM Purple Tomato is not destined solely for the fields of commercial agriculture—it has made its debut in the backyards of home gardeners across the United States.
With claims of heightened antioxidant levels and potential health benefits, this novel creation has stirred both excitement and controversy among consumers and scientists alike. Biotech investors hope it can usher in a new era of public trust in genetically engineered foods while skeptics worry the tomatoes’ near-total lack of regulation or review may hide dangers to human health and/or the environment.
Development
The GM Purple Tomato was engineered by scientists at Norfolk Plant Sciences in the UK. Led by biochemist Cathie Martin and her team, the project aimed to harness the natural properties of anthocyanins, compounds found in blueberries and blackberries, to enhance the nutritional profile of tomatoes.
Using genetic engineering techniques, Martin and her colleagues inserted two genes responsible for purple coloration in edible snapdragon flowers into tomato plants. This process enabled the tomatoes to express the genes from the snapdragon and, subsequently, produce high levels of anthocyanins, thereby imbuing the tomatoes with a distinct purple hue and potentially enhanced health benefits.
According to Norfolk Healthy Produce, the U.S. subsidiary of Norfolk Plant Sciences, the Purple Tomatoes are a “rich source of antioxidants” due to the increased content of anthocyanins. Unlike domesticated tomatoes which contain anthocyanins in the skin, the Purple Tomato contains anthocyanins throughout the whole tomato.
The genesis of the GM Purple Tomato marks a significant milestone in agricultural biotechnology. Unlike previous GM crops primarily targeted at commercial producers, this tomato is the first GM food crop directly marketed to home gardeners in the United States, offering an opportunity for individuals to engage with biotechnology in their own backyard.
According to Norfolk Healthy Produce, more than 13,000 Purple Tomato seed orders have already shipped.
Regulatory Approval
The GM Purple Tomato was deregulated by the U.S. Department of Agriculture (USDA) in 2022. According to a statement from the USDA, the GM Purple Tomato is not subject to regulation by the USDA because it does not pose a plant pest risk:
“With respect to Norfolk Plant Sciences’ purple tomato, we did not identify any plausible pathways to increased plant pest risk compared to other cultivated tomatoes and issued a response letter indicating the plant is not subject to regulation.”
In 2023, the Purple Tomato received a “no questions” letter from the U.S. Food and Drug Administration (FDA), which means the Purple Tomato is considered “generally recognized as safe” (GRAS) and, therefore, does not require premarket review or approval by the FDA.
To qualify for GRAS status, Norfolk Plant Sciences submitted data from tests conducted internally.
The lack of safety testing by the USDA and FDA, as well as reliance on data generated by the company that will profit from approval of its own product, has led to some experts calling for a more comprehensive safety assessment.
Safety Concerns and Health Claims
Data provided to the FDA by Norfolk Plant Sciences demonstrates the company conducted various safety tests. However, critics argue the tests are insufficient to guarantee the safety of the Purple Tomato for human consumption.
According to an FDA memo dated June 13, 2023, tests conducted by Norfolk Plant Sciences mainly focused on six areas. Of those, four were relatively straightforward while two have raised safety concerns among experts, according to GM Watch.
The Straight Forward Tests
1. PCR and Southern blot analysis were conducted by Norfolk Plant Sciences to determine if the snapdragon foreign DNA was inserted into the tomato DNA.
- The company (Norfolk Plant Sciences) stated that insertion of the foreign DNA was confirmed.
2. PCR and sequence comparison of DNA samples were conducted to confirm the stability of the inheritability of the insertion across generations. Plants were bred to determine if the purple phenotype was inherited in a Mendelian segregation fashion.
- The company stated the purple phenotype was inheritable.
3. Compositional analysis was conducted to determine if the Purple Tomato contained similar nutrients at similar levels compared with non-GMO tomatoes, including protein, fat, carbohydrate, fiber, minerals, carotenoids, vitamins, and alpha-tomatine.
- The company determined the levels of most of the nutritional components to be similar or with “minor differences.”
4. Norfolk Plant Sciences assessed dietary exposure levels assuming the complete replacement of red tomatoes in the human diet with the Purple Tomato for two days.
- The company concluded the level of dietary exposure to anthocyanins is the same as consuming high-anthocyanin foods. For example, 8 ounces of Purple Tomato juice is equivalent to consuming 1 cup of blueberries.
The Controversial Tests
1. Bioinformatic analyses were utilized to determine if any open reading frames were generated or disrupted by inserting the foreign DNA. Norfolk Plant Sciences searched the DNA sequences flanking the insertion sequence in the tomatoes.
- The company reported no open reading frames flanking the insertion location.
“There’s no evidence that the developers of the GM purple tomato have carried out the kind of molecular analyses (proteomics and metabolomics) that could help establish whether they only got the change they want, with no unintended changes. As a result, we don’t know if these tomatoes are safe to eat,” said Mr. Antoniou.
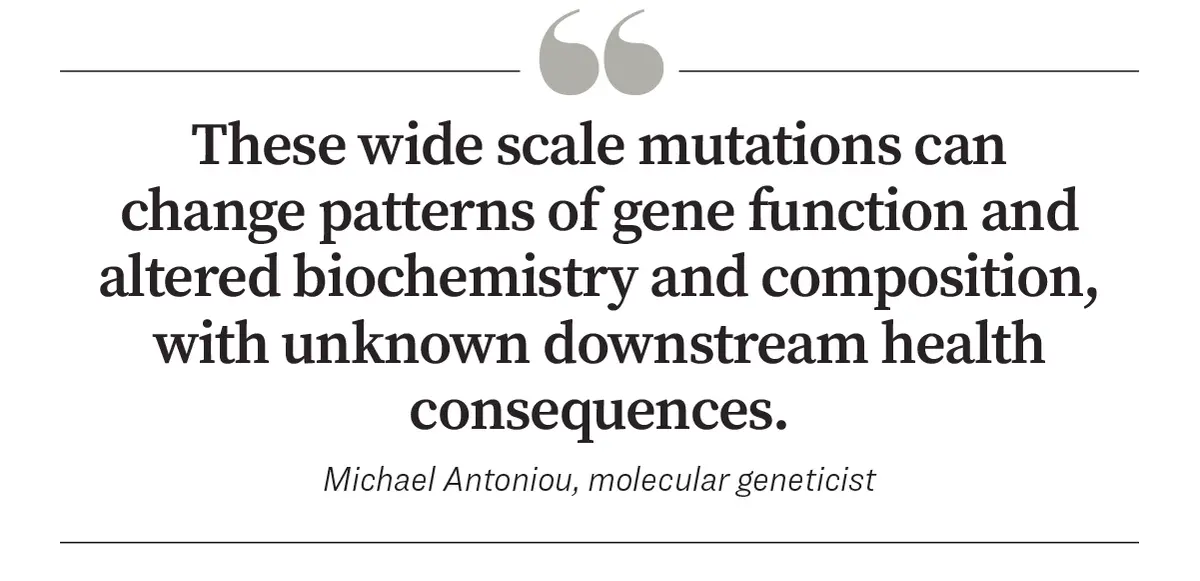
“We must also bear in mind that the GM transformation process (plant tissue culture and plant cells transformation) will inevitably give rise to hundreds if not thousands of sites of unintended DNA damage (mutations). These wide scale mutations can change patterns of gene function and alter biochemistry and composition, with unknown downstream health consequences,” he said.
Assessment was conducted on new peptides of 30 amino acids or more at the insertion site of foreign DNA to address concerns about toxicity or allergenicity. The company identified a “putative” peptide but stated that it had no similarity to known allergens or proteins and was not transcribed in tomatoes, concluding that there were no food safety concerns.
While allergenicity is a persistent issue in genetically modified foods, Norfolk Plant Sciences did not find any matches to known allergens in their assessment. However, it is important to note that this does not guarantee that the peptide produced through gene modification is not allergenic. Given the high prevalence of food allergies in the United States, caution should be exercised when altering the genetic composition of our food.
The study conducted by Norfolk Plant Sciences and Cathie Martin in 2008, which examined the impact of Purple Tomato supplementation on cancer-susceptible mice, showed that the GM tomatoes extended the mice’s lifespan by an average of 40 days compared to non-GM red tomatoes. This led to sensationalized media headlines about the potential cancer-fighting properties of the Purple Tomato.
Subsequent claims about the health benefits of the GM Purple Tomato, such as preventing cardiovascular disease and fighting cancer, have been refuted by the UK’s National Health Services, citing the lack of evidence from human studies. Excessive consumption of anthocyanin in the Purple Tomato may pose unexpected risks, as the benefits of antioxidants are not as straightforward as commonly believed.
Anthocyanins, like other nutrients, follow the principle of balance in the body. While they have potential health benefits, consuming too much can lead to adverse effects, including kidney, liver, and thyroid issues. Antioxidant supplementation has also been linked to an increased risk of cancer, highlighting the importance of moderation in nutrient intake.
Maintaining a balance of reactive oxygen species (ROS) is crucial for overall health, as they play a dual role in both combating disease and causing damage if levels are too high. The potential health risks associated with excessive anthocyanin consumption from GM Purple Tomatoes underscore the importance of understanding and monitoring nutrient intake to avoid unintended consequences. The content of anthocyanins in the GM tomato averages about 500mg/100g of fresh fruit, which is approximately 40 times more than the daily average consumption. This amount is significantly higher compared to other naturally purple colored fruits like sweet cherries, blackberries, strawberries, red raspberries, and black grapes, which contain anthocyanins in the range of 3-143mg/100g, up to 160-fold less than the GM purple tomato.
Mr. Antoniou expressed concerns about the potential health risks of ingesting high doses of antioxidants, such as anthocyanins, from the GM purple tomatoes. He warned that it could disrupt the delicate balance between too much and too little ROS, leading to negative health outcomes.
A possible solution to this issue is the use of traditionally bred alternatives. Traditional plant breeding techniques have already produced a variety of purple tomatoes with elevated levels of anthocyanins without the need for genetic modification. Varieties like Black Zebra and Black Beauty, along with the “Indigo” cultivars developed by Jim Myers at Oregon State University, offer nutrient-dense options for consumers.
While the GM Purple Tomato is marketed as a nutrient-dense food aimed at improving health, there are concerns about its potential long-term effects on human health and the environment. As the debate over GMOs continues, transparency, evidence-based decision-making, and public engagement are crucial in shaping the future of agriculture and our food supply. Please provide an alternative version of the text.
Source link